.
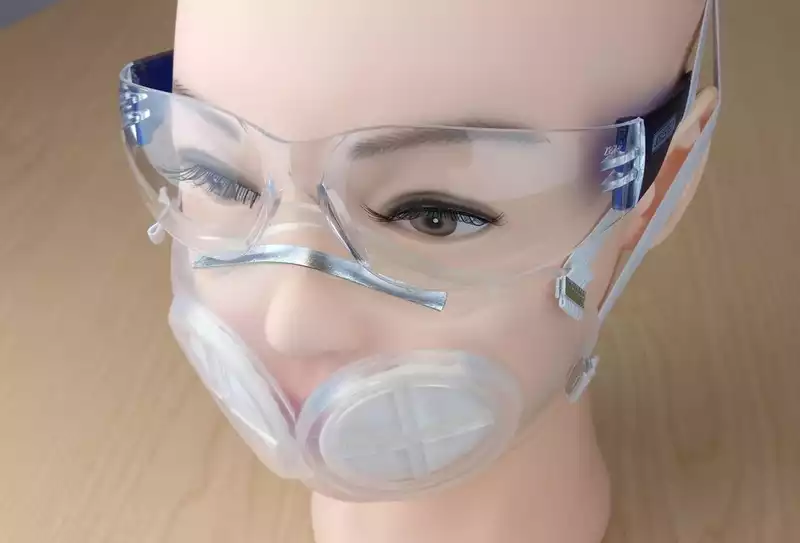
What is even easier is that the masks do not need to be sterilized using specialized equipment, as has been the case with previous N95 masks. Rather, iMASC (which stands for Injection Molded Autoclavable, Scalable, Conformable) need only be sterilized with steam, bleach or rubbing alcohol, or placed in an oven at a temperature that will kill bacteria and viruses.
As such, iMASC has the potential to revolutionize the way N95 masks are used. For starters, most iMASCs are reusable, thus reducing the environmental impact of wearing masks, especially disposable ones.
More importantly, however, the reusability of iMASCs means that the demand for N95 masks can be better managed. When the coronavirus pandemic hit the United States, the demand for N95 masks meant that many healthcare workers ran out of fresh disposable masks, forcing many to disinfect and reuse masks that would have been discarded after a certain number of uses and sterilization.
iMASC has the potential to solve this problem, especially if there is another spike in COVID-19 infections. iMASC not only allows for the reuse of safe and practical N95 masks for healthcare workers, but also for the general public, thus supply will not be eaten up.
In addition, at 15 pounds per mask for hospitals, iMASC is a more overall affordable alternative to disposable N95 masks, which range from $2.80 to $6.95 per mask, CNBC reports.
Before this can happen, however, more testing is needed to see if iMASC is suitable for widespread use.
iMASC also needs to be approved by the Food and Drug Administration (FDA) and the National Institute for Occupational Safety and Health. However, once this step is completed and iMASCs are ready to be manufactured on a large scale, they could transform the face of mask wearing in the U.S. and abroad.









Comments